March 13, 2018; revised April 18, 2020; August 28, 2022
1. Waves involve the transport of energy without the transport of matter. When you drop a pebble onto a water reservoir, you can see the ripples move out. There is no water displacement from one place to another, but the disturbance moves out.
- Therefore, a wave can be described as a disturbance that travels through a medium, transporting energy from one location (its source) to another without transporting matter.
- On the other hand, a particle can move and transfer matter. The most important characteristic of a particle is that its position is localized at any given time and is detected as a single detection event or a “single click” on the detector. That means a “whole particle” arrived at the detector.
- Those are the ways waves and particles were expected to behave before the advent of quantum mechanics. But starting around 1900, our ideas about waves and particles became somewhat confusing due to many drastic changes that took place over many years.
2. The fundamental concepts in quantum mechanics (QM) were worked out roughly from 1900 to 1930. Andrew Whitaker gives a good description of the evolution of QM within this period and beyond in his book “Einstein, Bohr and the Quantum Dilemma” (second edition, 2006).
- That book describes how the keywords like waves, particles, and wave functions related to QM evolved. Some of the old — and unnecessary — concepts like “wave-particle duality” linger because of the impressions made at that time.
- Experiments carried out within the past 20-30 years (some key experiments within the past few years) show that such lingering ideas on “wave-particle duality” are an obstruction to grasping the reality revealed by QM.
3. For a long time, the light was thought to be a wave, specifically an electromagnetic wave. That idea still lingers on.
- Light consists of particles (photons.) That was firmly established only in 1986. See “Photons Are Particles Not Waves.”
- The most distinguishing characteristic of a particle is that its detection is recorded as a single event (“a click”) at the detector.
4. However, the motion of a particle — including a photon — can be represented by a wave function, which is a mathematical function, not a wave. A wave function is extremely useful for calculating experimental results but does not have a physical reality. Rather it represents the physical reality.
- It is easier to see the differences among the terms waves, particles, and wave functions by looking at what happens when waves and particles go through two adjoining slits.
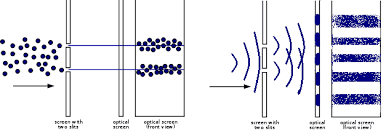
5. When normal particles that we are familiar with go through two slits and fall on a screen to make their imprints, we will see two “line images,” as shown on the left in the figure below. On the other hand, a wave (like a water wave) will give rise to “fringes,” as shown on the right.
- In normal life, we will see particles (say marbles) going through two large slits leading to those marbles hitting the screen, as shown on the left.
- With a water wave going through two slits, we will see ripples giving rise to water wave crests, as shown on the right.
Those are the scenarios with normal particles and normal waves.
6. If quantum particles (like electrons or photons) are going through two slits where slit openings are LARGE (say a cm or more), then we will again see the “normal particle pattern” shown on the LEFT.
- However, if quantum particles (like electrons or photons) are going through two slits where slit openings are SMALL (say less than an mm), then we will see the “wave pattern” shown on the RIGHT. If the aperture dimensions are of the order of h/p (where h = Planck’s constant and p is the particle’s momentum), then such diffuse wave patterns can be expected.
- Those experimental results can be CALCULATED in such cases by using wave functions to represent the motion of such particles.
- However, a particle is never spread out. A given particle will always be detected at a certain point within that diffraction pattern. One must repeat the experiment with a single particle many times to get that diffraction pattern.
7. April 18, 2020: Here is a youtube video that explains the above:
We will discuss the above and related issues in detail in this section: “Quantum Mechanics and Dhamma.”
Light is a Wave or a Particle?
8. Newton’s concept of light consisting of particles prevailed for a long time in the early days. But Newton’s corpuscular theory of light was abandoned around 1850 because it could not explain interference and diffraction phenomena. Young and Fresnel showed that the wave picture could explain those experimental results.
- However, a wave needs a medium to support it. A “water wave” propagates in water, and a sound wave can propagate in a solid or a liquid and needs at least air to propagate. Still, light can travel in a vacuum, so the existence of a yet unknown “aether” was proposed as the all-pervading medium through which light could propagate.
- The “ether theory” itself ran into several objections and was finally abandoned after the famous Michelson–Morley experiment performed in 1887 conclusively proved the absence of an aether.
9. Now we know that light doesn’t need a medium through which to travel. Furthermore, the speed of light is constant. It is independent of the movement of the source or detector or the direction in which it travels, as shown by Einstein’s theory of relativity (discovered in 1905).
- Therefore, light is not a wave. This was confirmed by an experiment conducted with single photons in 1986, which we will discuss in the next post. I just wanted to present the background in this post.
Matter as Waves?
10. While the debate was going on about whether light is a wave or a particle between the 1850s to early 1900s, and even up to 1986 to some extent, another related development came with the early studies in quantum mechanics beginning around 1900.
- The issue was whether solid particles could be treated as waves.
11. After Planck, Einstein, Compton, and others established that light behaved as particles (photons), in 1913, Bohr came up with an idea to quantize the energy levels of a hydrogen atom. He was able to explain why discrete lines in the spectra of hydrogen.
- Yet another groundbreaking hypothesis by de Broglie in 1924 clarified why Bohr’s idea worked. He proposed that just like photons can be represented by a wave (specifically with electromagnetic wave equations of Maxwell), a “wave can represent the motion of electrons.” At that time, it was not clear what this “wave” would be. Now, we know that it is a wave function.
12. Light has been considered a wave for a long time, as discussed above. But the idea that waves could represent electrons with no-zero rest mass was unanticipated.
- Then in 1927, Davisson and Germer produced clear diffraction patterns for electron scattering from a nickel lattice, just like a diffraction pattern due to light. This led to the speculation that maybe particles sometimes behave as waves.
- That is how the idea of “wave-particle duality” evolved from 1900 to about 1930. Even though an accepted “quantum theory” was established around 1930, the idea of “wave-particle duality” lingers to the present.
- Nowadays, those diffraction patterns seen with electrons can be explained via the wave functions representing electrons’ motion. However, a given electron can be found only at one location at a given time.
Heisenberg Uncertainty Principle
13. To make things even more complicated, in 1927, Heisenberg came up with his famous uncertainty principle. This principle says that the uncertainty of the position of a particle (σx) multiplied by the uncertainty of the particle’s momentum ( σp) must be larger than what is known as Planck’s constant, ℏ:
σx . σp ≥ ℏ
- Planck’s constant is extremely small; it has a value of about 10-34 Js.
- For any particle that we can see with our eyes, any uncertainty in the particle’s position will be much smaller than the size of the particle. Therefore, we don’t notice this in our normal lives.
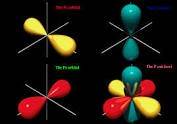
14. However, when it comes to microscopic particles like electrons, the position uncertainty is usually huge. If you have seen a pictorial representation of the orbit of an electron in a hydrogen atom, it is shown as an area; the electron could be anywhere within that area.
The following picture shows some examples of such electron orbitals. An electron could be anywhere within a given orbital at a given time.
- Therefore, the critical point is that the uncertainty in a particle’s position and momentum (or velocity) becomes significant only for small particles like electrons and photons.
15. We can make the following statements about the location of such a “quantum particle” at a given time.
- The significance of this uncertainty is that we cannot say precisely where such a small particle is to be found. We can only say that it should be located within a particular region. We can calculate the probability of finding it at a given point within that region.
- But that does not mean “the particle is spread out in that volume.” At any given time, the particle is located at only one point. It is just that we cannot say precisely at which point due to the uncertainty principle.
16. I hope you can see the difference. Some people make the grave mistake of saying a quantum particle is “spread over space” like a wave. That is a grave mistake and a key reason people have difficulty understanding quantum mechanics.
Any questions on these QM posts can be discussed at the discussion forum: “Quantum Mechanics – A New Interpretation.”